In the oil and gas industry, corrosion-related failures have been reported to constitute over 25% of total safety incidents. The problem of corrosion in oilfield environments is predominantly associated with the presence of corrosive species such as CO2 (causing sweet corrosion) and H2S (causing sour corrosion), and other associated reservoir constituents. Oxygen-related corrosion is mostly encountered in water injection systems. Some of the corrosion is found associated with as-manufactured defects on metallic components. This corrosion leads to material degradation causing costly failures with severe consequences for human life and the environment [1]–[3].
According to NACE IMPACT, in 2013, the global cost of corrosion-related economic losses for all industries is estimated to be USD 2.5 trillion, which is equivalent to 3.4% of the global Gross Domestic Product (GDP) including corrosion in the oil and gas production industry where the total annual cost of corrosion is estimated to be USD 1372 billion [1], [4].
Commonly, corrosion control focuses on materials and environments. Active corrosion control is by taking effective control of the process of corrosion, which includes the modeling of the system, material selection, and general design. The material selection process is one of the key stages in the integration assurance of the system, which plays an important role in the overall corrosion control process [1], [5].
Materials Selection Consideration
Choosing among materials to manage the corrosion needs a very careful understanding of the working and environmental conditions including design pressure and temperature, weldability, compatibility, and corrosion concerns especially the severity of the service fluid, such as pH, H2O, H2S, CO2, O2, salt, and other fluid content of the crude oil or gas. The materials selection process shall consider all statutory and regulatory requirements. The project design criteria, such as design lifetime, cost profile (CAPEX/OPEX), inspection and maintenance philosophy, safety and environmental profiles, operational reliability, failure risk evaluations, and other specific project requirements should be considered [5]–[7].
In addition, other criteria such as the safety and easy-to-repair level should be considered in the material selection process. As a rule of thumb, it should be taken extra attentions to products that are used in hard-to-repair locations. For offshore installations and particularly subsea, access for the purposes of maintenance and repair can be limited and costly and should be carefully considered in the design [5]–[7].
Material Selection Methods
The selection of materials can be a complex procedure, if improperly carried out can lead to mistakes in application and misunderstanding about the performance of a materials in a specific service environment. There are a variety of ways individuals and companies select materials [8]:
- Companies with large research facilities typically initiate a test program that involves simulating the particular part of the field environment under study (i.e. flowlines versus downhole). Based on the available information, a group of alloys is selected that represents a possible range of alternatives. It is more cost-effective and less time-consuming to test only a few materials that are likely candidates, rather than test all alloys all the time [8].
- Review the literature for corrosion data that generally applies to the anticipated field conditions. This can narrow the number of candidate alloys for testing. The selected materials are then tested under very specific conditions to fill gaps in literature data and/or field experience. Care must be taken when using this approach, for example, the corrosion resistance of many materials is specific to a particular temperature range. Likewise, changes in critical environmental components such as elemental sulfur can have a profound impact on the resistance to stress corrosion cracking (SCC), and another important factor in alloy selection [8].
- The quickest and least expensive alloy selection method is simply to review the codes, standards, and literature including but not limited to ISO 21457 [6], NORSOK M-001 [7], and ISO 15156/NACE MR0175 (all parts) [9], [10], and existing or similar field data, and make the selection. These standard covers different alloy types with separate tables for different applications. It is important to obtain the latest version and corrigenda. This method can be quite unsatisfactory since certain critical factors or conditions will not be known and must be assumed. A greater chance for error exists in this approach is the introduction of a potential for failure of the materials or the use of a more expensive alloy than is required. It is advisable if this method is used, to consult with someone who has a working knowledge of materials and their applications [8].
Materials Selection Framework
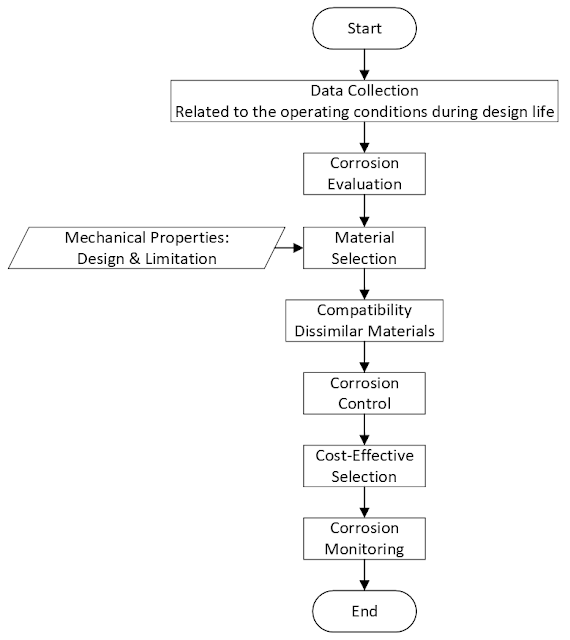
This article provides an overview of material selection and corrosion protection for the oil and gas industry based on well-established and robust material selection guidelines standards including ISO 21457 [6], NORSOK M-001 [7], and ISO 15156/NACE MR0175 (all parts) [9], [10]. Figure 1 shows the material selection framework. This article gives an overview of guidance and requirements for:
- Corrosion evaluation.
- Design and limitations in mechanical properties
- Material selection based on codes, standards, and literature.
- Compatibility of dissimilar materials.
- Corrosion protection and control.
- Cost-effective selection.
- Corrosion monitoring.
Corrosion Evaluation
Corrosion evaluation is an inevitable part of the material selection process. It is necessary to know the environment of oil and gas in the system. Commonly, sweet service or sour service. ISO 15156/NACE MR0175 defines ‘sweet service’ as service in any gas free of hydrogen sulfide. While ‘sour service’ is used to identify environments containing measurable amounts of H2S [10]. Production of sand and other solids are also common in most oil and gas wells. Therefore, materials selection in oil and gas is normally based on an evaluation of corrosion and erosion. The corrosion mechanisms and the specified process design parameters are included in ISO 21457, for offshore installations, NORSOK M-001 should be considered, see Table 1 and Table 2 [5]–[7].
All internal and external media should be considered for the entire design life. This should also include the stages of transportation, storage, installation, testing, and preservation. The aim of corrosion evaluation is to find how corrosive and aggressive the service fluid is to the materials. Corrosion evaluations and calculations should be based on well-known corrosion models (such as the Sell model, NORSOK M-506, etc.), laboratory tests, or field experiences [5]–[7].
Calculated corrosion rates based on models should be verified by the end user in order to incorporate field experience. If corrosion evaluation using the field experiences method, requires a well-documented successful experience of the material usage at the specific service fluid condition for at least two years and should preferably involve a full examination of the equipment following field use. In this case, according to ISO 15156/NACE MR0175, the usage of the same material at a similar service condition is acceptable. The severity of intended service conditions shall not exceed that of the field experiences [5]–[7], [10].
Design and Limitations in Mechanical Properties
Mechanical properties such as yield strength, tensile strength, hardness, and impact toughness should be considered in the selection of materials. In addition, for parts of the production system that operate at low temperatures or experience low temperatures, can suffer thermal cooling stresses and thus require material toughness to be sufficient to prevent brittle fracture under these conditions [6], [7], [9], [10].
Exposure temperatures during intermediate stages, such as manufacturing, storage, testing, commissioning, transport, and installation, should be considered when specifying the design temperature. Material properties based upon the minimum and maximum design metal temperatures and wall thicknesses shall be in compliance with specified design codes. The material weldability should also be considered to ensure an effective fabrication [6], [7], [9], [10].
The following guidelines for design and limitations in mechanical properties should apply for materials selection [6], [7], [9], [10]:
- The specified minimum yield strength (SMYS) of carbon and low alloy steels intended for welding should not exceed 560 MPa. A higher SMYS may be specified, provided that documentation showing acceptable properties with respect to weldability and the properties of the base material, heat-affected zone, and weld metal is presented.
- Usage limitations for materials in H2S-containing environments shall be in accordance with ISO 15156-2 and ISO 15156-3.
- Free-machining steel grades should not be used.
- Austenitic SS castings with PREN ≥ 40 should not be used for butt weld components due to risk of micro cracking in the heat-affected zone (HAZ) in weldments.
- For martensitic carbon, low-alloy and CRA, the hardness of any components shall not exceed 328 HB or 35 HRC (submerged parts that exposed to CP).
- Ferritic-austenitic (duplex) stainless steel shall be regarded as potentially susceptible to HISC, independent of SMYS or specified maximum hardness. Compliance with DNV-RP-F112 shall be specified (submerged parts that exposed to CP).
- The hardness of weld and HAZ of any steel grade should not exceed 350 HV10 for non-H2S-containing service.
- Titanium should not be used for hydrofluoric acid or anhydrous methanol with water content less than 5 %.
- Certain titanium alloys in submerged parts may not be compatible with cathodic protection.
Materials Selection Based on Standards
Nowadays, codes like ISO 21457 [6], NORSOK M-001 [7], and ISO 15156/NACE MR0175 (all parts) [9], [10] provide materials selection guidelines for specific systems and areas in the oil and gas industry. Based on these codes [6], [7], [9], [10], for wet hydrocarbon systems, the internal and external corrosion mechanisms indicated in Table 1 and Table 2 should be evaluated. Materials carbon steel or corrosion-resistant alloy (CRA) listed in Table 1 and Table 2 are the recommended options for material selection in this systems. CRA should be evaluated against carbon steel when this is considered appropriate in the materials selection process [6], [7].
However, these standards do not exclude the use of other materials. Use of materials with equal or better performance shall be agreed with end-user [6], [7]. Furthermore, materials selection for other systems and areas such as oil and gas production and processing systems, flare systems, produced water systems, injection systems, utility systems, pipelines, and flowlines are provided in ISO 21457 [6] and NORSOK M-001 [7].
In addition, the possibility for "sour" service conditions during the lifetime shall be evaluated. Sour service definition, metallic materials' requirements, and qualification shall be according to ISO 15156/NACE MR0175 (all parts) [6], [7], [9], [10]. The evaluation and use of materials in conditions containing H2S, where cracking including hydrogen-induced cracking (HIC) shall follow the requirements. This evaluation should include the potential for future changes in reservoir H2S content. Dehydration of gas or use of corrosion inhibitors should not relax the requirement to use H2S-resistant materials [6], [7], [9], [10].
Material selection shall be optimized and provide acceptable safety and reliability for the entire design life. As a minimum based on code and standard [6], [7], [9], [10], the following shall be considered:
- Corrosivity, taking into account specified operating conditions including start up and shut-down conditions.
- Design life and system availability requirements.
- Failure probabilities, failure modes and failure consequences for human health, environment, safety, and material assets.
- Resistance to brittle fracture.
- Inspection and corrosion monitoring.
- Access and philosophy for maintenance and repair.
- Minimum and maximum operating temperature.
- Minimum and maximum design temperature.
- Weldability (girth welds and overlay welds).
- Hardenability (carbon and low alloy steels).
- Priority shall be given to materials with good market availability and documented fabrication and service performance.
- Number of different materials shall be minimized considering stock, costs, interchangeability and availability of relevant spare parts.
- Environmental impact and authority permissions, e.g., on discharge of chemicals like corrosion inhibitors shall be considered.
|
Corrosion mechanism |
Description |
Parameters |
Materials prone |
|
|
CS & LAS1 |
CRA2 |
|||
|
CO2
and H2S corrosion |
CO2
corrosion is one of the most common corrosion mechanisms oil and gas
production and processing systems. The presence of H2S in
combination with CO2 influences the corrosion. The type of
corrosion is dependent on the proportions of these constituents in the
production fluids. |
Temperature,
partial pressure and fugacity of CO2 and H2S, pH,
content of organic acids and flow conditions. |
Yes |
Yes3 |
|
MIC |
Microbiologically
induced corrosion (MIC) caused by living organisms such as bacteria, algae,
or fungi. It is often associated with the presence of tubercles or slimy
organic substances. Often, the bacteria produce localized corrosion in the
form of pitting or crevice corrosion. |
Water
intermittent, stagnant, or low-flow conditions allow and/or promote the
growth of microorganisms. pH range of 0 to 12, and temperatures from 0 °F to
235 °F. |
Yes |
Yes |
|
SSC/SCC
caused by H2S4 |
Sulfide
Stress Cracking (SSC) is the cracking of a susceptible metal under the
combined action of tensile stress and corrosion in the presence of water and
H2S. SSC occurs in high-strength (high-hardness) steels, in highly
localized zones of high hardness in weld metal and HAZs. PWHT is beneficial
in reducing the high hardness and residual stresses. |
H2S
level, pH, contaminants, temperature, microstructure, hardness (correlates to
strength), and tensile stress level (applied or residual). |
Yes |
Yes |
|
HIC/SWC4 |
Hydrogen-Induced
Cracking (HIC) results from hydrogen atoms diffusing into the steel as a
result of corrosion, resulting in internal separations parallel to the
surface of the steel. The separations grow then eventually forming a
thru-wall leak path. Interconnecting cracks between HIC separations on
different planes have a stair-step appearance, so HIC is sometimes referred
to as “Stepwise Cracking (SWC)”. |
See
SSC/SCC caused by H2S, with consideration that blistering and HIC
damage develop without applied or residual stress. PWHT will not prevent them
from occurring. |
Yes |
No |
|
ASCC5 |
Alkaline
environments containing compounds such as amines, caustic or carbonates can
cause alkaline stress corrosion cracking (ASCC) of carbon steels, especially
where there is the potential to concentrate these compounds, e.g. in the
presence of crevices or evaporation. Typical
mitigation measures may include heat treatment after welding or forming, use
of protective coatings, and CRA. |
Level
of tensile stress and/or residual stress, the type and concentration of
amines, NH3 and H2S, caustic or carbonates,
temperature, pressure, fluid velocity, localized turbulence, pH, and alloy
composition. |
Yes |
No |
|
SCC
without H2S |
Internal
Stress Corrosion Cracking (SCC) can occur in SS in the absence of H2S
and dissolved oxygen. This is due to water evaporation and
deposition/concentration of chlorides in high salinity waters and at high
temperatures. Systems where this can occur should be designed with fresh wash
water injection facilities or resistant materials should be used. |
Chloride
content, temperature, pH, tensile stress, presence of oxygen, and alloy
composition. |
No |
Yes |
|
Erosion/Erosion-Corrosion |
The
material loss due to flowing solid particles (sand) or in a liquid or vapor
stream physically abrading the material to material loss accelerated by the
flow of corrosive liquid or vapor possibly combined with the
velocity-assisted removal of a protective film or scale. |
The
velocity and number of impacting particles (sand), size, shape, hardness, and
density of particles, the hardness of the material, and the angle of impact. |
Yes |
Yes |
|
1) CS = Carbon Steel; LAS = Low Alloy
Steel 2) CRA = Corrosion-Resistant Alloy 3) The presence of H2S in
combination with CO2 can also lead to localized attacks of CRAs.
There are no generally accepted limits and the limits vary with type of CRA. 4) The evaluation and use of materials
in conditions containing H2S, where cracking including
hydrogen-induced cracking is possible, shall follow the requirements given in
ISO 15156/NACE MR0175 (all parts) 5) Reference can be made to
NACE RP0403 for guidance on caustic cracking or to API RP 945 for guidance on
amine cracking. |
||||
|
Corrosion mechanism |
Description |
Parameters |
Materials prone |
|||||||||||||
|
CS & LAS1 |
CRA2 |
|||||||||||||||
|
External
marine atmospheric environments |
The
external marine atmospheric environment contains water and chloride salts.
Carbon steel will be corroded. CRAs can suffer from corrosion, including
pitting, crevice corrosion and SCC. Welds are particularly vulnerable to SCC. A
coating may be applied to prevent such corrosion and SCC. Operate material
below Maximum Operating temperature (MOT) limits to avoid chloride-induced
SCC. Nickel-,
titanium- and copper-based alloys are in general considered to be immune to
SCC in marine environments. |
MOT
limits to avoid chloride-induced SCC:
|
Yes |
Yes |
||||||||||||
|
Buried
and submerged installations |
The
corrosion of metals from exposure to soils and submerged environment. The
external protection of buried or submerged structures is usually achieved by
a combination of external coatings and cathodic protection. Depending
on metallurgical condition, some CRAs, such as UNS S17400, UNS N05500 and
duplex stainless steel, can be susceptible to HSC while catholically
protected. |
Temperature,
moisture and oxygen availability, soil/water resistivity, soil/water type,
cathodic protection, stray current drainage, and coating type, age, and
condition. |
Yes |
Yes |
||||||||||||
|
Corrosion
Under Insulation (CUI) and fireproofing |
Corrosion
of piping, pressure vessels, and structural components resulting from water
trapped under insulation or fireproofing.
|
Temperature,
presence of chlorides, local condensation on the metal surface, duration of
wetting, coating system, insulation and sealing system, and environment. |
Yes |
Yes |
||||||||||||
|
1) CS = Carbon Steel; LAS = Low Alloy
Steel 2) CRA = Corrosion-Resistant Alloy |
||||||||||||||||
Corrosion-Resistant Alloy (CRA) Selection Literature
Compatibility of Dissimilar Materials
- Flange connection is the preferred method of connecting dissimilar materials when there is a significant risk for internal galvanic corrosion.
- Install a distance spool between the dissimilar metals so that they will be separated by at least 10 pipe diameters from each other. The distance spool may be either of a solid electrically non-conducting material (e.g. GRP) or of a metal that is coated internally with an electrically non-conducting material, e.g. vulcanized rubber. The metal in the distance spool should be the most noble of the dissimilar metals unless vulcanized rubber lining is selected.
- Apply a corrosion allowance on the less noble metal or a sacrificial thick-walled carbon steel spool, which is designed for replacement at scheduled intervals.
- Install internal sacrificial anodes through access fittings near the interface, e.g. resistor-controlled cathodic protection for seawater systems.
- Apply electrical isolation of dissimilar metals; however, the risk of electrical continuity via pipe supports, decks and earthing cables should be evaluated.
- At critical interfaces between dissimilar metals in hydrocarbon production and processing systems, weld overlay of the flange face of the less noble material with a corrosion-resistant material may be considered.
- Apply a non-conducting coating on the most noble of the dissimilar metals to reduce the cathode area. The coating shall extend at least 10 pipe diameters into the most noble pipe material.
- For connections between copper alloys and stainless steel/nickel alloys/titanium, the use of easily replaceable spools with added wall thickness should be evaluated.
- Direct contact between aluminum and carbon steel or copper alloys shall be prevented.
- If the use of dissimilar metals is unavoidable and necessary, an attempt to select metals which form “compatible couples or groups” should be made. The “Galvanic Corrosion Indicator” published by the International Nickel Company Ltd. can be useful [15].
Corrosion Protection and Control
1. Chemical Treatment
2. Corrosion Allowance (CA)
- 1.0 mm to 1.5 mm for non-corrosive service.
- 3.0 mm for mildly corrosive service.
- 6.0 mm for severely corrosive service.
3. Internal and External Coating
4. Cathodic Protection (CP)
A. Offshore
B. Onshore
5. Splash Zone Protection
6. Corrosion Protection of Closed Compartments
7. Corrosion Under Insulation Control
8. Other Corrosion Controls
Cost-Effective Selection
- Total installation cost.
- Service life.
- Maintenance cost.
- Time and cost requirement to replace or repair at the end of service life.
- Cost of downtime to replace or repair.
- Cost of inhibitors, extra facilities, or training required to assure achievement of predicted service life.
- Time value of money.
- Factors which impact taxation, such as depreciation and tax rates.
- Inflation rate.
Corrosion Management
- Definitions of roles, responsibilities and reporting routines within the organization.
- Corrosion risk evaluation.
- Planning and execution (methods, location and frequency) for corrosion monitoring, process parameter monitoring and water analyses.
- Planning and execution of addition of corrosion control chemicals.
- Develop procedures for evaluation of corrosion monitoring data and for verification that the corrosion rates and conditions are within acceptable levels (pre-defined targets).
- Definition of consequences and actions if targets are not met.
1. Corrosion Monitoring
|
Method |
Applicable systems |
Comments |
Notes |
|
Weight
loss coupon |
All
systems |
Coupon
should be of the same/similar material as the wall. May include weld |
1 |
|
Linear
polarization resistance |
Systems
with an aqueous/electrically conducting phase |
Requires
normally approx. 30% aqueous phase with min. 0.1% salinity |
|
|
Galvanic
probes |
Aqueous |
Water
injection systems |
|
|
Electrical
resistance |
All
systems |
Downstream
inhibitor injection points when monitoring pipelines |
|
|
Erosion/sand
monitoring probes |
Process
flowline systems Subsea
production systems |
|
|
|
Hydrogen
probes |
Hydrocarbon
systems |
For
sour service conditions |
|
|
Notes
1.
Recommended maximum time between inspection/replacement: 3 months. |
|||